Abstract
Background: About two-thirds of AML patients harbor mutations in signaling and kinase pathways (e.g., FLT3, KRAS, NRAS, PTPN11, NF1, KIT), which utilize the growth factor receptor bound protein-2 (Grb2) to induce AML progression. Prexigebersen (BP1001), a liposome-incorporated Grb2 antisense oligonucleotide that inhibits Grb2 expression, suppresses cancer growth and survival. A Phase I/IB single center study (Ohanian, Lancet Haematol 2018) in relapsed/refractory AML patients demonstrated BP1001 safety up to 90 mg/m 2 and demonstrated CR/CRi in 3 of 6 relapsed/refractory AML patients treated with BP1001 + low dose cytarabine combination in the Phase IB portion. Preclinical studies indicated that BP1001 enhanced the inhibitory effects of decitabine (DAC), or DAC + venetoclax (VEN) against AML cells. Thus, we designed a clinical trial with three treatment arms: (1) BP1001 + DAC + VEN for newly diagnosed AML, (2) BP1001 + DAC + VEN for relapsed/refractory AML, (3) BP1001 + DAC for relapsed/refractory AML patients who are VEN-resistant or -intolerant [ClinicalTrials.gov Identifier: NCT02781883]. The study was amended to obtain safety run-in data for patients treated with BP1001 + DAC first before proceeding to safety run-in for patients treated with BP1001 + DAC + VEN. Here we report the safety run-in and efficacy data of AML patients treated with BP1001 + DAC or BP1001 + DAC + VEN.
Methods: This is a multi-center open-label, Phase II study in newly diagnosed and relapsed/refractory AML patients to determine the safety and preliminary efficacy of BP1001 (given at 60 mg/m 2 over 2-3 h twice weekly for a total of 8 doses over a 28-day cycle) in combination with DAC (20 mg/m 2, once daily for 5 days) or in combination with DAC and VEN (400 mg, once daily). Eligible patients were considered unsuitable for or refused intensive chemotherapy and had ECOG performance status of 0-2. Patients treated with at least 1 cycle of therapy were evaluable for safety run-in.
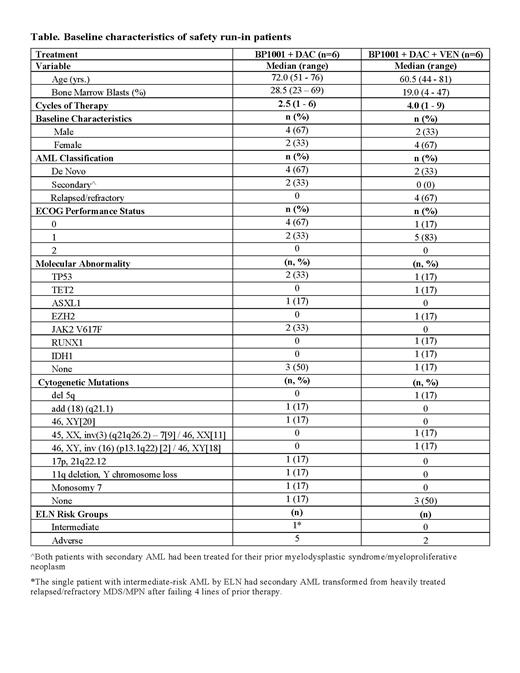
Results: Between April 2018 and May 2020, 6 patients (4 male: 67%) with median age 72 years (range, 51 - 76) were treated with at least 1 cycle of BP1001 + DAC combination therapy. Four patients (67%) had de novo AML and two (33%) had secondary AML. All patients in this cohort were considered high risk due to having either adverse risk status by ELN (n=5) or treated secondary AML (n=1) (transformed from myelodysplastic syndrome/myeloproliferative neoplasm after failing 4 lines of prior therapy). Adverse events (AEs) were generally consistent with those expected with DAC and/or AML and included diarrhea (67%), fatigue (50%), constipation (50%), nausea (50%), dyspnea (50%), hypoxia (50%), hypotension (50%) and pain in extremity (50%). The most frequent serious AE (SAE) was bacteremia (33%). Two de novo patients (33%) achieved a CRi and 1 treated secondary AML patient (17%) achieved a PR. Patients received a median of 2.5 cycles of therapy (range, 1 - 6); median duration of follow-up was 140 days (range, 72 - 678).
Since May 2020, 6 patients (2 male: 33%) with median age 61 years (range, 44 - 81) were treated with at least 1 cycle of BP1001 + DAC + VEN combination therapy. Two (33%) had de novo AML, and four (67%) were relapsed/refractory. All patients in this cohort were adverse-risk by ELN (n=2) or relapsed/refractory (n=4). AEs were generally as expected with DAC, VEN and/or AML, including fatigue (67%), diarrhea (67%), constipation (67%), decreased appetite (67%), hypokalemia (67%), hypocalcemia (50%), edema peripheral (50%), asthenia (50%), nausea (50%), abdominal pain (50%), dysphagia (50%), hypotension (50%) and febrile neutropenia (50%). The most frequent SAEs were sepsis (33%) and febrile neutropenia (50%). Four patients (67%) achieved a CR/CRi/MLFS (n=1/2/1) and 1 (17%) achieved a PR. Of these patients, 3 were relapsed/refractory (1 CR/1 CRi/1 MLFS) and two were de novo (1 CRi/1 PR). Patients received a median of 4 cycles of therapy (range, 1 - 9); median duration of follow-up was 177 days (range, 113 - 318).
Conclusions: BP1001-based combination therapy has been safely administered to high-risk and relapsed/refractory AML patients who were considered unsuitable for standard chemotherapy. Preliminary data showed the combination treatment of BP1001 + DAC or BP1001 + DAC + VEN was well-tolerated, with encouraging efficacy signals. The study will continue enrollment across all three cohorts. Drug dosing for the study will proceed as planned.
Lin: AbbVie, Aptevo Therapeutics, Astellas Pharma, Bio-Path Holdings, Celgene, Celyad, Genentech-Roche, Gilead Sciences, Incyte, Jazz Pharmaceuticals, Novartis, Ono Pharmaceutical, Pfizer, Prescient Therapeutics, Seattle Genetics, Tolero, Trovagene: Research Funding. Ritchie: Abbvie: Consultancy, Honoraria; Takeda: Consultancy, Honoraria; Incyte: Consultancy, Honoraria, Speakers Bureau; ARIAD Pharmaceuticals: Ended employment in the past 24 months, Speakers Bureau; Novartis: Consultancy, Honoraria, Other: travel support, Research Funding, Speakers Bureau; Celgene/BMS: Consultancy, Other: travel support, Speakers Bureau; Astellas: Consultancy, Research Funding; Bristol Myers Squibb: Consultancy, Research Funding; NS Pharma: Research Funding; Protaganist: Consultancy, Honoraria; Jazz: Consultancy, Research Funding; Pfizer: Consultancy, Research Funding. Pemmaraju: Clearview Healthcare Partners: Consultancy; ASH Communications Committee: Membership on an entity's Board of Directors or advisory committees; Plexxicon: Other, Research Funding; MustangBio: Consultancy, Other; Roche Diagnostics: Consultancy; Springer Science + Business Media: Other; DAVA Oncology: Consultancy; Stemline Therapeutics, Inc.: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other, Research Funding; Abbvie Pharmaceuticals: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other, Research Funding; Celgene Corporation: Consultancy; LFB Biotechnologies: Consultancy; Protagonist Therapeutics, Inc.: Consultancy; HemOnc Times/Oncology Times: Membership on an entity's Board of Directors or advisory committees; Samus: Other, Research Funding; Daiichi Sankyo, Inc.: Other, Research Funding; Novartis Pharmaceuticals: Consultancy, Other: Research Support, Research Funding; Affymetrix: Consultancy, Research Funding; Blueprint Medicines: Consultancy; Cellectis S.A. ADR: Other, Research Funding; Aptitude Health: Consultancy; CareDx, Inc.: Consultancy; Dan's House of Hope: Membership on an entity's Board of Directors or advisory committees; Sager Strong Foundation: Other; ASCO Leukemia Advisory Panel: Membership on an entity's Board of Directors or advisory committees; Incyte: Consultancy; Bristol-Myers Squibb Co.: Consultancy; ImmunoGen, Inc: Consultancy; Pacylex Pharmaceuticals: Consultancy. Borthakur: Ryvu: Research Funding; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees; University of Texas MD Anderson Cancer Center: Current Employment; Astex: Research Funding; Protagonist: Consultancy; ArgenX: Membership on an entity's Board of Directors or advisory committees; GSK: Consultancy; Takeda: Membership on an entity's Board of Directors or advisory committees. Tari Ashizawa: Bio-Path Holdings, Inc.: Current Employment, Current equity holder in publicly-traded company, Membership on an entity's Board of Directors or advisory committees. Kantarjian: Immunogen: Research Funding; Ipsen Pharmaceuticals: Honoraria; AbbVie: Honoraria, Research Funding; Amgen: Honoraria, Research Funding; Daiichi-Sankyo: Research Funding; Aptitude Health: Honoraria; Precision Biosciences: Honoraria; Novartis: Honoraria, Research Funding; Taiho Pharmaceutical Canada: Honoraria; NOVA Research: Honoraria; Astra Zeneca: Honoraria; Ascentage: Research Funding; BMS: Research Funding; Astellas Health: Honoraria; Pfizer: Honoraria, Research Funding; Jazz: Research Funding; KAHR Medical Ltd: Honoraria. Cortes: Novartis: Consultancy, Research Funding; Sun Pharma: Consultancy, Research Funding; Takeda: Consultancy, Research Funding; Bristol Myers Squibb, Daiichi Sankyo, Jazz Pharmaceuticals, Astellas, Novartis, Pfizer, Takeda, BioPath Holdings, Incyte: Consultancy, Research Funding; Bio-Path Holdings, Inc.: Consultancy, Membership on an entity's Board of Directors or advisory committees; Pfizer: Consultancy, Research Funding. Roboz: Amgen: Consultancy; MEI Pharma - IDMC Chair: Consultancy; Bayer: Consultancy; AstraZeneca: Consultancy; AbbVie: Consultancy; Celgene: Consultancy; Blueprint Medicines: Consultancy; Glaxo SmithKline: Consultancy; Agios: Consultancy; Helsinn: Consultancy; Jazz: Consultancy; Janssen: Consultancy; Astex: Consultancy; Bristol Myers Squibb: Consultancy; Actinium: Consultancy; Jasper Therapeutics: Consultancy; Novartis: Consultancy; Otsuka: Consultancy; Mesoblast: Consultancy; Daiichi Sankyo: Consultancy; Astellas: Consultancy; Janssen: Research Funding; Pfizer: Consultancy; Roche/Genentech: Consultancy.